Registration for the November 2024 course will open shortly.
Studies based on Mendelian randomization are increasingly being used to distinguish causal relationships from observational associations in epidemiology and to prioritize potential targets for pharmaceutical intervention. This course intends to explain both simple and more complex statistical methods for causal inference in Mendelian randomization studies, and the instrumental variable assumptions on which they are based. The course includes several computing practicals in R.
Intended audience
Course materials offer an introduction to Mendelian randomization techniques and are relevent to causal inference in both epidemiology and drug development. The course is particularly suitable for PhDs and post-docs about to start projects using Mendelian randomization, such as medical/applied/pharmaceutical statisticians, and quantitative epidemiologists.
Some familiarisation with R and RStudio is needed to fully appreciate the practicals, but introductory material will be included with the pre-reading for the course.
Course structure
Overview
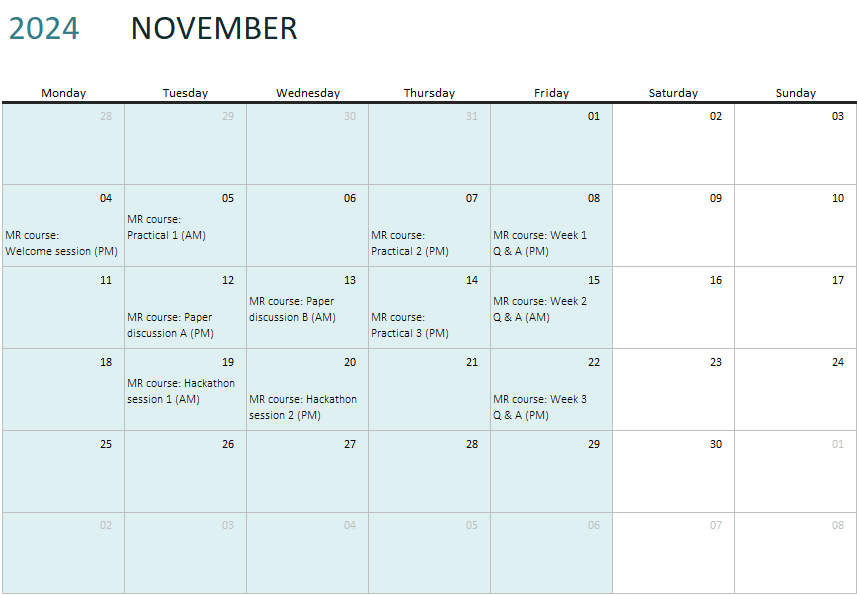
The course consists of four half-days worth of content plus the final hackathon, and will take place over 3 weeks, although participants will have access to the course material for the duration of the month of the course (and usually for some time after the course). Each half-day consists of some on-demand pre-recorded content and some timetabled (live) content. The first day of Week 1 will be Monday 4th November 2024. All the core content of the course is pre-recorded - live sessions are not compulsory to attend, but are supplementary to the core content. They represent a chance to engage with the course tutors. Several of the live sessions will be recorded.
Each half-day can be done whenever is convenient. A half-day of content includes three pre-recorded talks (around 20-30 minutes each) and one practical session (around 1-1.5 hour). Each of the three computer practicals should be performed individually, but there is an associated live drop-in session to come and ask questions. There is also a recorded debrief session that runs through the practical content. In terms of live content, in addition to the practical drop-in sessions, paper discussion, and hackathon, there is also a question and answer session each week to ask your questions. Questions can be asked during the week on a dedicated Slack channel, or you can ask questions live (Q+A sessions will be recorded).
The hackathon is an opportunity to perform your own Mendelian randomization investigation to use the skills you have gained during the course. This can be done individually or as part of a group.
Participants should choose one of the papers for the timetabled paper discussion (Practical 3), and one hackathon session, based on their availability and preference.
On demand content
On-demand content.
Week 1 on demand content
Half day 1
- Talk 1: Motivation and introduction to Mendelian randomization
- Talk 2: Estimating a causal effect using individual-level data
- Talk 3: Some examples of Mendelian randomization
Practical 1: Mendelian randomization using individual-level data
Half day 2
- Talk 4: Summarized data and two-sample MR
- Talk 5: Assumptions of MR
- Talk 6: Statistical issues in MR
Practical 2: Individual-level and summarized data
Week 2 on demand content
Half day 3
- Talk 7: Robust methods with summarized data
- Talk 8: Choosing instruments for MR
- Talk 9: Practical implementation
Practical 3: Discussion of published MR papers
Half day 4
- Talk 10: Multivariable MR
- Talk 11: Interpretation of MR estimates
- Talk 12: MR for drug effects
Practical 4: Summarized data and software packages
Week 3 on demand content
Hackathon example
Timetabled content
Timetabled content
Morning sessions start at 9.30 UK time, afternoon sessions start at 14.00 UK time. We do not expect all attendees to be present for every session.
Q+A sessions will be recorded, drop-in sessions act in an "office hour" format, and participants should choose one of each of the paper discussion and hackathon sessions.
For further information about the course, please contact: